Medical Device Regulation (MDR) is a regulation in the European Union that sets out the essential requirements for medical devices. These requirements are intended to ensure that medical devices are safe, effective, and perform as intended. A medical device manufacturer must demonstrate that their device meets these requirements before it can be placed on the market.
An MDR essential requirements checklist template can help manufacturers to ensure that they have considered all of the essential requirements and that their device meets these requirements. An essential requirements checklist template can be a valuable tool for medical device manufacturers. It can help to ensure that their devices meet the requirements of the MDR and that they are safe and effective for use.
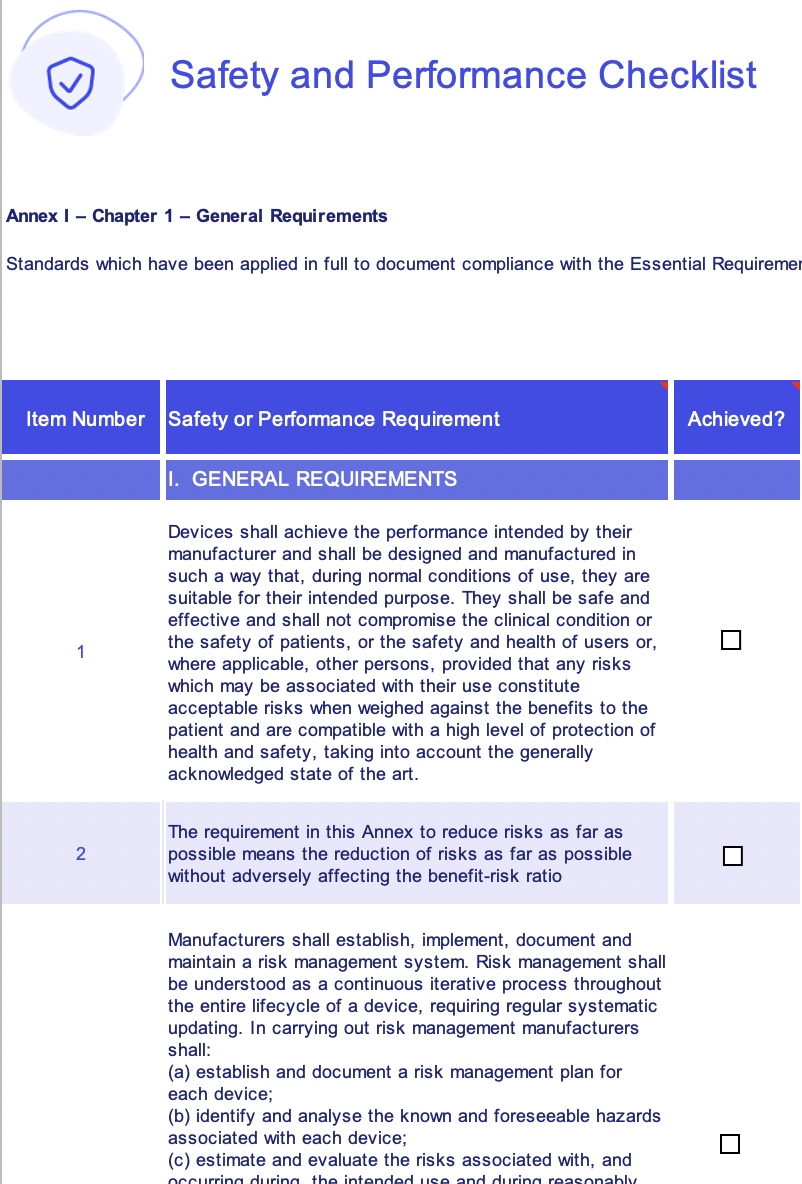
Essential Requirements of the MDR
The MDR essential requirements are divided into two categories: general requirements and specific requirements. The general requirements apply to all medical devices, while the specific requirements apply to specific types of medical devices.
The general requirements include:
- The device must be safe and effective for its intended purpose.
- The device must be manufactured in accordance with good manufacturing practices.
- The device must be labeled and packaged in a way that ensures its safe use.
- The device must be accompanied by instructions for use that are clear and understandable.
The specific requirements depend on the type of medical device. For example, the specific requirements for implantable medical devices include:
- The device must be designed and manufactured to minimize the risk of infection.
- The device must be biocompatible with the human body.
- The device must be able to withstand the stresses of implantation and use.
Benefits of Using an MDR Essential Requirements Checklist Template
There are many benefits to using an MDR essential requirements checklist template. These benefits include:
- It can help manufacturers to ensure that they have considered all of the essential requirements.
- It can help manufacturers to identify any areas where their device does not meet the essential requirements.
- It can help manufacturers to develop a plan to address any deficiencies.
- It can help manufacturers to avoid delays in the regulatory approval process.
Conclusion
An MDR essential requirements checklist template is a valuable tool for medical device manufacturers. It can help to ensure that their devices meet the requirements of the MDR and that they are safe and effective for use.
Manufacturers who use an MDR essential requirements checklist template are more likely to be successful in the regulatory approval process and to bring their devices to market quickly and efficiently.